in December 2019, a large outbreak of the novel coronavirus disease (COVID–19) occurred in Wuhan, China, and rapidly spread to other countries. This new class of coronavirus, known as severe acute respiratory syndrome coronavirus (SARS-CoV-2), can cause acute respiratory infections and is easily transmitted from person to person. Therefore, a simple and rapid serological test is needed to detect positive cases.
the high rate of transmission of COVID-19 has forced people to stay at home until it is possible to prevent further spread of the disease. Due to the large number of asymptomatic cases and because there have not been many epidemiological studies in this case; Investigation of infection rate and effective epidemic control is required.
In this situation, the quick and correct diagnosis of this disease is very important to prevent the epidemic and save people’s lives. Based on this, various diagnostic tests based on molecular and serological detection methods have been developed, some tests and diagnostic kits are offered in the market. Among them, detection methods are widely used, which is the preferred test for SARS-CoV-2 detection.
Despite the good results of detecting COVID-19 by RT-PCR method, this test is time-consuming, expensive and requires trained personnel and specialized equipment. In fact, there are many cases that have clinical symptoms or specific chest tomography (CT) results but are not diagnosed by this method.
this is due to variable viral concentrations, different types of samples, different sampling methods, different sampling times (early or late stages of infection), preservation and transportation of samples, different genetic targets, etc
serological tests that measure a significant increase in specific IgG and IgM antibodies in patients’ serum are good complementary tests to RT-PCR tests for the diagnosis of COVID-19 patients and are very valuable in assessing the immune status of individuals.
in addition, by using serological data, important epidemiological information can be collected and the disease status can be monitored more reliably. In fact, finding specific antibodies against the virus plays an important role in vaccine and drug production processes. In addition, these tests are quick, economical, and easy to perform and do not require trained personnel or special equipment.
IgM antibodies appear 7-10 days after the onset of symptoms and usually decrease after 20 days, until they disappear. IgG antibody becomes positive from day 14 to 20 after the onset of symptoms and is usually maintained for a long time.
Therefore, this diagnostic method is useful for evaluating the treatment process as well as vaccination and safety after that. Accordingly, the presence of IgM in the patient’s serum means a new infection or the first stage of infection. If both IgM and IgG antibodies are positive, the patient is in the second stage of infection. If only IgG is positive, the patient is in the last stage of the disease or it means that the person has been sick for some time in the recent past. However, it should be noted that since the rise of antiviral antibodies occurs about 7 days after infection, serological tests are not suitable tests for early detection of the virus, while they are very useful for prevention and further management of the disease. be The graph of the increase and decrease of immunoglobulins from the beginning of the infection is shown in image 1
The rapid detection kit for IgM and IgG antibodies of the new coronavirus (COVID-19) of Arvin Bio Health Company is used for the qualitative detection and differentiation of IgM and IgG antibodies in serum, plasma (EDTA, citrate) or whole blood.
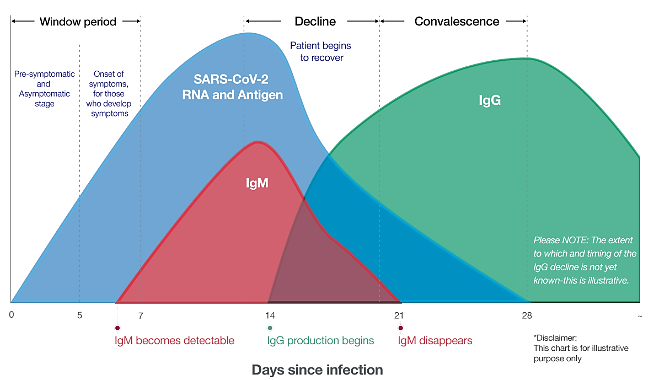
Image 1: Chart of increase and decrease of immunoglobulins from the beginning of infection
. Boes, Marianne. 2000. ‘Role of natural and immune IgM antibodies in immune responses’, Molecular immunology, 37: 1141-49.
2. Broughton, James P, Xianding Deng, Guixia Yu, Clare L Fasching, Venice Servellita, Jasmeet Singh, Xin Miao, Jessica A Streithorst, Andrea Granados, and Alicia Sotomayor-Gonzalez. 2020. ‘CRISPR–Cas12-based detection of SARS-CoV-2’, Nature Biotechnology: 1-5.
3. Chan, Jasper Fuk-Woo, Cyril Chik-Yan Yip, Kelvin Kai-Wang To, Tommy Hing-Cheung Tang, Sally Cheuk-Ying Wong, Kit-Hang Leung, Agnes Yim-Fong Fung, Anthony Chin-Ki Ng, Zijiao Zou, and Hoi-Wah Tsoi. 2020. ‘Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens’, Journal of clinical microbiology, 58.
4. Fang, Yicheng, Huangqi Zhang, Jicheng Xie, Minjie Lin, Lingjun Ying, Peipei Pang, and Wenbin Ji. 2020. ‘Sensitivity of chest CT for COVID-19: comparison to RT-PCR’, Radiology: 200432.

